Glaucoma Vision Restoration: What's New in January 2026
Glaucoma Vision Restoration: What’s New in January 2026 Glaucoma is often called the “silent thief of sight” – a group of eye diseases where damage to...
시각 건강을 유지하기 위한 심층 연구 및 전문가 가이드.

Glaucoma Vision Restoration: What’s New in January 2026 Glaucoma is often called the “silent thief of sight” – a group of eye diseases where damage to...

Introduction Glaucoma is a common eye disease that damages retinal ganglion cells (RGCs) – the nerve cells that carry visual signals from the eye to t...
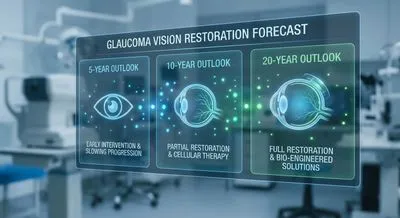
Forecasting Glaucoma Vision Restoration: 5-, 10-, and 20-Year Outlook Glaucoma causes progressive loss of the retinal ganglion cells (RGCs) that send...
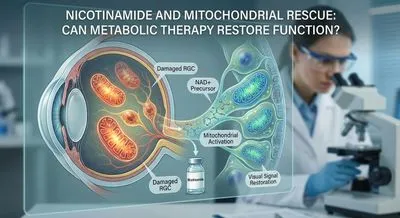
Nicotinamide and Mitochondrial Rescue: Can Metabolic Therapy Restore Function? Glaucoma is a leading cause of irreversible vision loss, often progress...

Aging, Senescence, and Glaucoma Glaucoma is a leading cause of blindness and its risk rises with age. In aged eyes, cells can enter a senescent state...
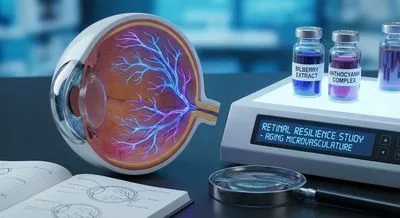
Anthocyanins and Bilberry Extracts: Retinal Resilience and Aging Microvasculature The flavonoids anthocyanins (pigments in berries) have long been cla...
Clinical trials are carefully planned research studies that test whether a medical treatment, device, or procedure is safe and effective in people. They follow strict rules and are coordinated by scientists, doctors, and regulators to protect participants and produce reliable results. Trials usually progress in phases: early phases check safety and dosing, while later phases compare the new intervention to standard care or a placebo to see if it works better. Common methods include random assignment, where participants are placed into groups by chance, and blinding, where patients and sometimes researchers do not know who received which treatment. Researchers measure predefined outcomes, such as symptom improvement, side effects, or biological markers, to determine benefit and risk. Before a trial starts, ethical review boards must approve the plan, and participants give informed consent to join with a clear understanding of possible risks and benefits. Clinical trials are essential because they provide the evidence that medicines and devices need to be approved and recommended. Even after approval, ongoing studies and monitoring keep checking for rare side effects or long-term outcomes, so well-conducted trials are the most reliable way to bring new medical advances into safe patient care.